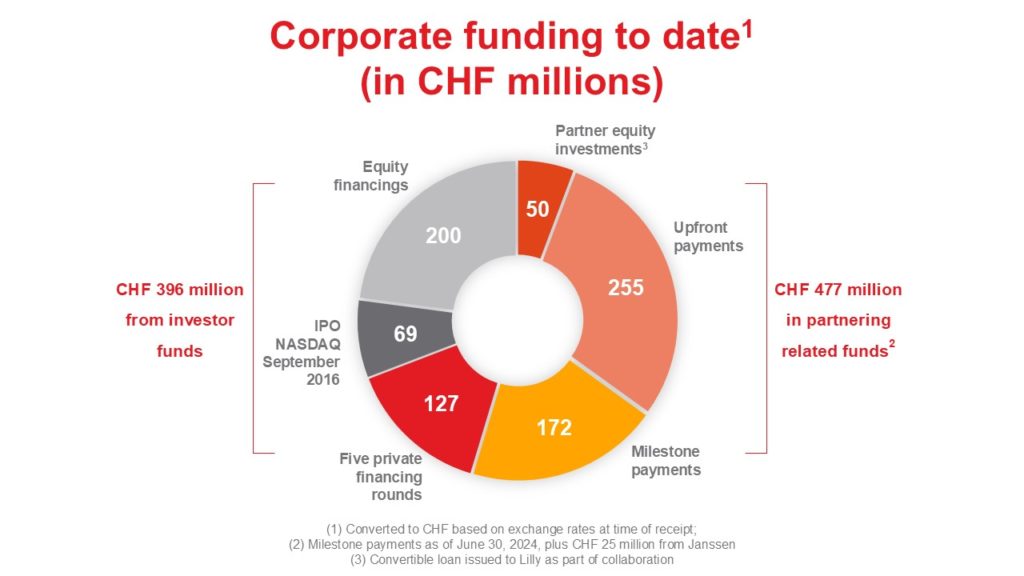
At AC Immune, we seek to partner our product candidates at the right times, opportunistically capturing optimal value for our shareholders while balancing risk. Our partnering strategy:
- reduces our investors’ exposure to risk, by partially monetizing assets at attractive deal terms
- accelerates time to market by accessing external resources
- preserves significant upside in the form of milestones and royalties
- retains development and marketing rights for specific indications.